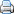Scientists increase lithium-sulfur battery lifetime by a factor of 10
February 25, 2013 by Lisa Zyga Enlarge
(a) TEM image of the sulfur cathode before discharge. The lithium sulfide (dark) is bonded to the inner wall of the hollow nanofiber (transparent). (b) TEM image of the sulfur cathode after full discharge. The lithium sulfide has shrunk away from the carbon wall, resulting in a loss of electrical contact and capacity decay. (c) TEM image of the polymer-modified sulfur cathode before discharge. (d) TEM image of the polymer-modified sulfur cathode after full discharge. The lithium sulfide remains attached to the carbon wall, improving capacity retention. Credit: Guangyuan Zheng, et al. ©2013 American Chemical Society
(Phys.org)—The world of rechargeable batteries is full of trade-offs. While lithium-ion (Li-ion) batteries are currently the most commercially successful, their low energy density doesn't allow for a long driving range. They are also very expensive, often accounting for half the price of electric vehicles. One alternative is lithium-sulfur (Li-S) batteries, which are attractive for their high gravimetric energy density that allows them to store more energy than Li-ion batteries. And although they still use some lithium, the sulfur component allows them to be much cheaper than Li-ion batteries. But one of the biggest drawbacks of Li-S batteries is their short cycle life, which causes them to lose much of their capacity every time they are recharged.
Ads by Google Graphene To Soar In 2013 - One "Breathrough Event' will send graphene to an all-time high - MoneyMorning.com/Graphene_Investing
Now a team of researchers led by Yi Cui, a professor of materials science and engineering at Stanford University, has developed a Li-S battery that can retain more than 80% of its 1180 mAh/g capacity over 300 cycles, with the potential for similar capacity retention over thousands of cycles. In contrast, most Li-S batteries lose much of their capacity after a few tens of cycles.
To achieve this improvement, the researchers first identified a new mechanism that causes capacity decay in Li-S batteries after cycling. In order for a Li-S battery to successfully recharge, the lithium sulfide in the cathode must be bound to the cathode surface—in this case, the inner surface of the hollow carbon nanofiber that encapsulates it. This binding creates a good electrical contact to allow for charge flow. But the researchers found that, during the discharge process, the lithium sulfide detaches from the carbon, resulting in a loss of electrical contact that prevents the battery from fully recharging. Before now, it has been very challenging to study the sulfur cathode at the nanoscale due to the sulfur compound's sensitivity to air and moisture, as well as its tendency to sublime under a vacuum. But the hollow carbon nanofiber structure of the anode—which the researchers developed in a previous study—protects the sulfur, which allowed the researchers to view the cathode using a transmission electron microscope (TEM) without significantly damaging the sample.
Read more at: http://phys.org/news/2013-02-scientists-lithium-sulfur-battery-lifetime-factor.html#jCp



 Desert Falcon's Archives
Desert Falcon's Archives